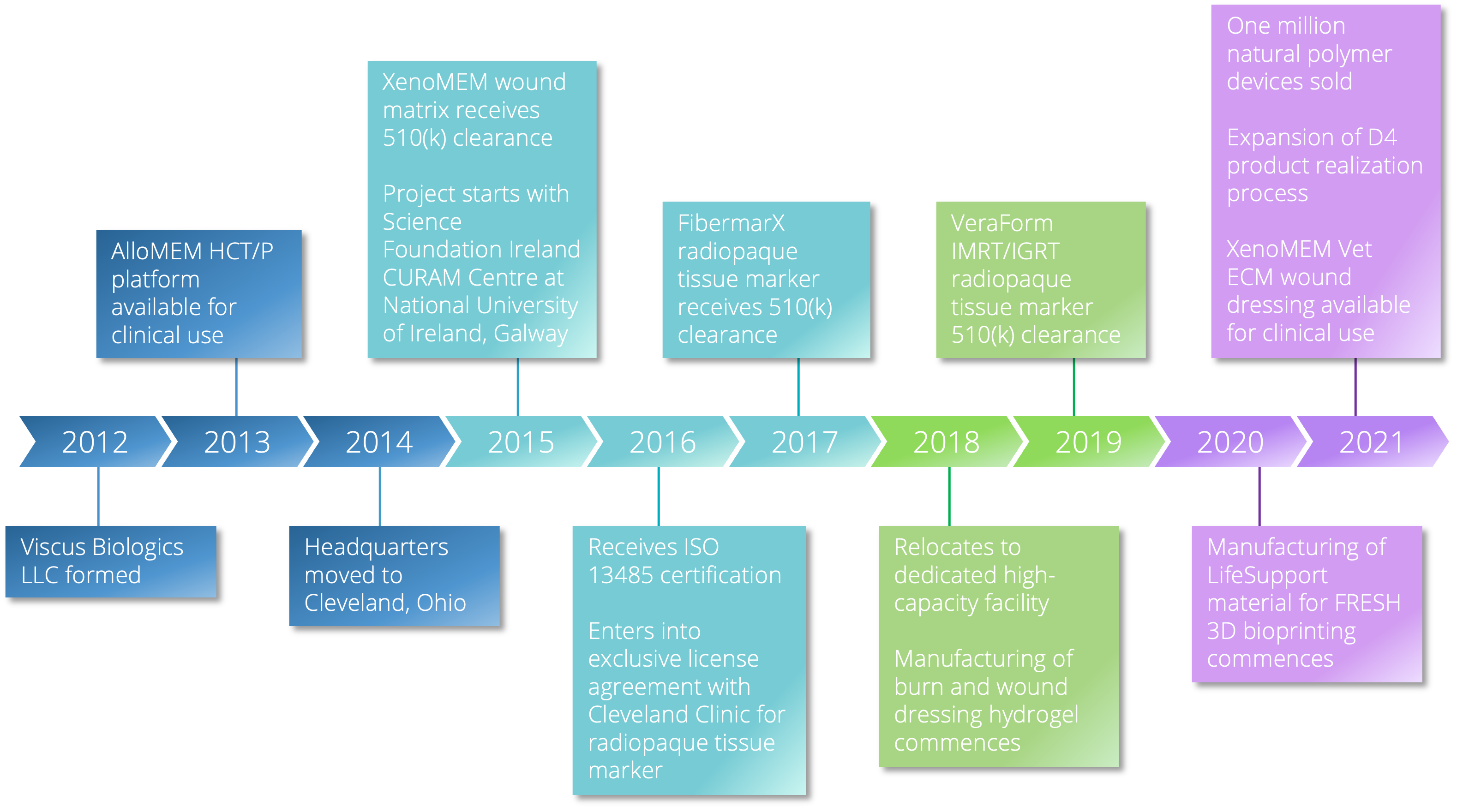
Viscus Biologics LLC specializes in helping people in the life science market segment using its established D4 process. Viscus Biologics is FDA registered with expertise in processing high performance biomaterials under a design services and contract manufacturing business model. Native polymers are processed into components, devices, and products. Several FDA cleared devices are produced in a controlled clean room setting.
Founded in 2012 to help companies solve complex challenges ranging from ideation to high volume production. We operate in centers of excellence for manufacturing and health care delivery.

World Headquarters
Cleveland, Ohio

Pre-Clinical Research
Dublin, Ireland
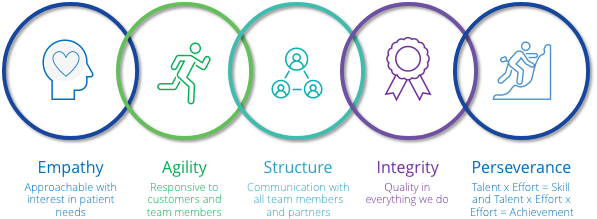
The culture at Viscus Biologics emanates from core values shared by team members throughout the organization.



Viscus Biologics, LLC designs, manufactures, and delivers innovative medical devices and life science products with the objective of positively impacting the health and well-being of patients. Given the nature of these products, Quality is of paramount importance in the culture and operation of the organization. We are committed to complying with FDA 21 CFR Part 820 and ISO 13485 to ensure safe and effective devices while maintaining and improving the effectiveness of our Quality Management System and providing a framework for the establishment and review of our quality objectives.
We are dedicated to unparalleled service to our customers, producing innovative and reliable medical devices, components, and life science products through our quality culture, engagement with our customers, training of our employees, and implementation of our strategic vision with partners.





